Guided Pharma Q/A & Compliance Teams Through Escalation Assessment & Serious Breach Evaluation in Veeva QMSCopy Link
Get a Demo
Overview
This pharmaceutical company faced challenges in identifying and addressing quality issues and potential serious breaches in regulatory compliance systematically and promptly. By implementing Whatfix’s in-app guidance on Veeva QMS, it improved process adherence, minimized compliance risks, and enhanced operational efficiency.
Preview
The Problem
Quality issues and potential serious breaches of regulatory requirements were not consistently identified or addressed in a timely manner, leading to compliance risks, quality concerns, and a lack of adherence to Veeva QMS processes. This resulted in operational inefficiencies, legal liabilities, and risks to the company's reputation due to potential fines from non-compliance.
The Whatfix Solutions
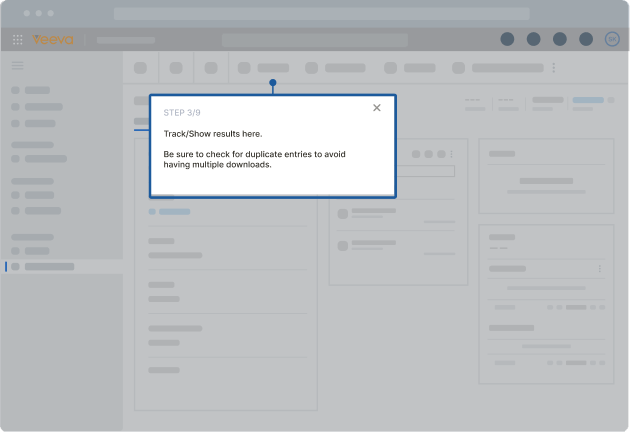
Whatfix provided step-by-step guidance through Flows and Smart Tips, assisting quality teams in choosing the correct record ID and escalation criteria based on issue types. Smart Tips throughout the Veeva QMS workflow served as field validations to guide users through critical steps in the quality assessment process, including determining the escalation level, selecting the attendee list for notifications, populating product impact, tracking results, and deciding if a Serious Breach (SB) Evaluation was necessary. If an SB was not required, the document was sent to the Assessment Outcome Approver for final approval.
Value on Investment
50% reduction in quality assurance errors (rework and rejections).
50% improvement in compliance with Veeva QMS processes.
Improved handling of quality issues, minimizing production delays and operational costs, ensuring a smooth supply chain, higher product quality, and continuous improvement.
Looking to experience Whatfix for yourself? Go for it.
Request a Whatfix Demo
