Reduced Medical Equipment QA Errors With In-App Smart Tips and Field Validations on Trackwise QMSCopy Link
Get a Demo
Overview
A medical equipment manufacturing company faced challenges with errors in Electronic Medical Device Reporting (eMDR) on Trackwise, leading to rejections by the FDA and increased costs. By implementing Whatfix’s in-app Smart Tips and Field Validations, the company reduced errors, improved report quality, and enhanced overall productivity.
Preview
The Problem
Employees needed to file complex eMDR reports on Trackwise, a quality management application, for internal review before submission to the FDA. These reports required data entries that depended on inputs from previously filled fields, and while some fields were non-mandatory, they still needed to be filled according to FDA guidelines. Despite internal reviews, critical errors were often missed, leading to frequent rejections by the FDA and substantial time and cost spent on re-filing the reports.
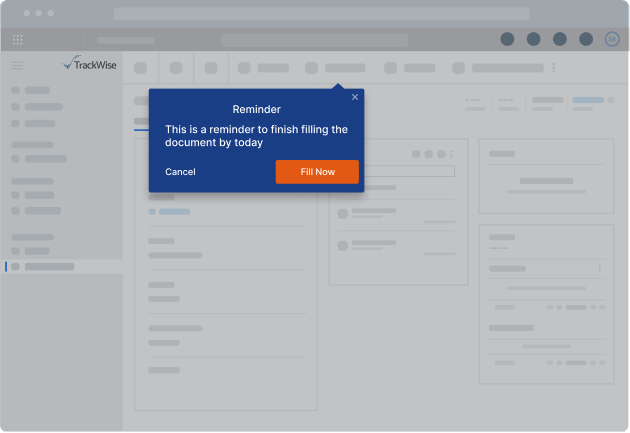
The Whatfix Solutions
Whatfix implemented Intelligent Smart Tips to nudge QA users to complete key fields that could not be left blank or required specific data formats. Users were blocked from submitting forms when certain fields were left incomplete. Additionally, Pop-Up notifications served as reminders for users with approaching deadlines or delays in submitting forms, ensuring timely and accurate submissions.
Value on Investment
Reduction in the number of reports rejected by the FDA.
Time and cost savings by minimizing user errors in the application.
Improved user productivity through enhanced guidance and fewer errors.
Looking to experience Whatfix for yourself? Go for it.
Request a Whatfix Demo
